
The AndraStent® Aortic is indicated for Native and / or recurrent Coarctation of the aorta in children, adolscents and adult patients. The CoCr (Cobalt-Chromium) allows a comparatively small introducer sheath even in the bigger sizes of the bare metal stent. A large selection of different lengths in two different models does offer a broad range of possible sizes to meet the patient needs.
The balloon-expandable AndraStent® Aortic can be re-dilatated due to somatic growth up to the maximum diameter, depending on the selected model XL or XXL.
To ensure a safe delivery of the AndraStent®, we recommend to use our AndraBalloon® or Andra2Balloon as Stent-Delivery Catheter.
The AndraStent® is CE marked under MDD 93/42/EEC since 2007 and in transition to MDR 2017/745 (NB 2409).
AndraStent® Aortic XL
Small introducer sheath 7F - 13F
- Diameter 8 - 25 mm
- Lengths 13 - 57 mm
- We recommend to use our AndraBalloon® or Andra2Balloon as Stent-Delivery-Catheter
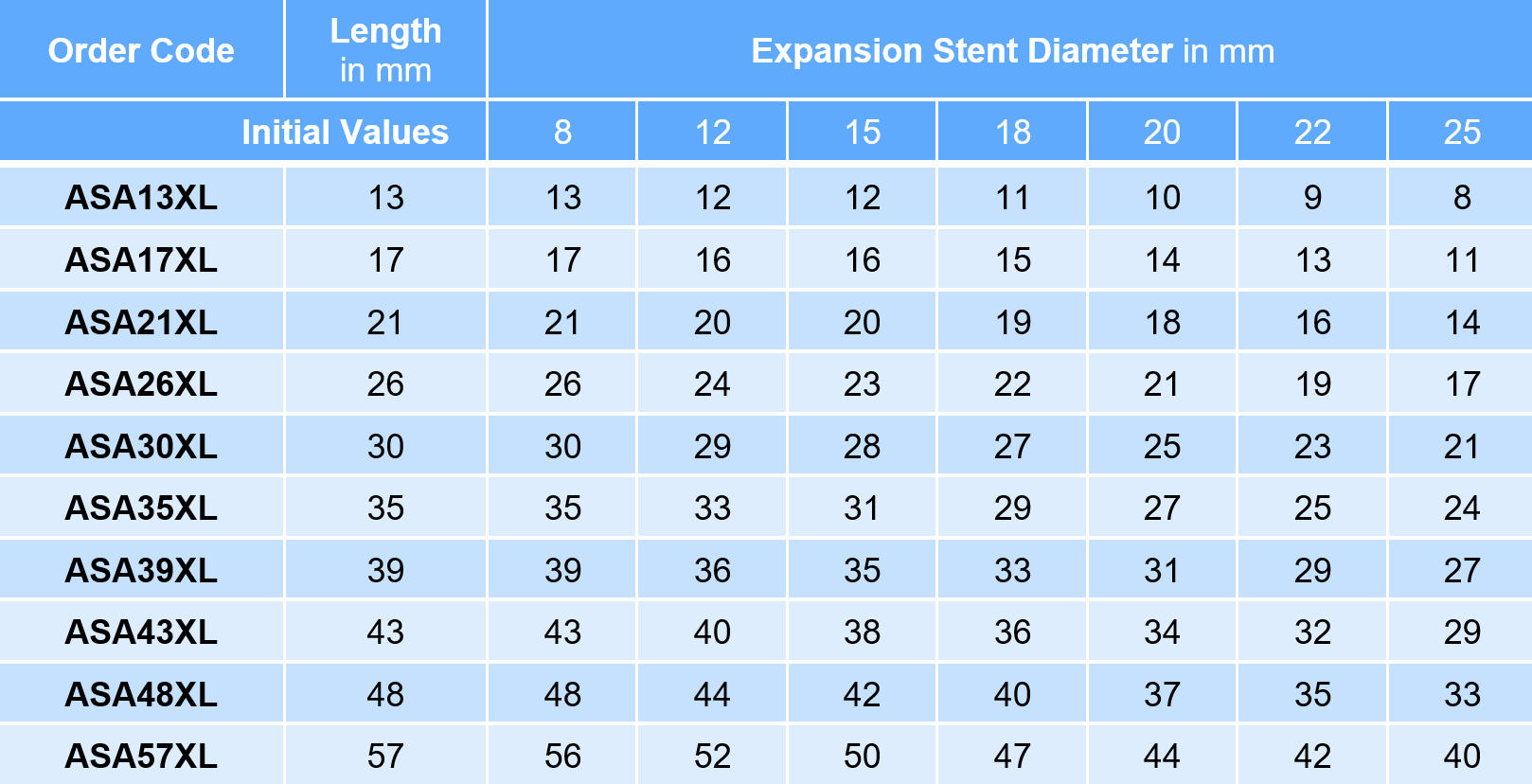
The diameter of the introducer sheath depends on the balloon (+ 1F for the stent).
Packaging unit of the AndraStent® Aortic XL: 1
AndraStent® Aortic XXL
Recommended introducer sheath 10F – 14F
- Diameters 12 – 32 mm
- Lengths 21 – 57 mm
- Biggest stent on the market for coarctation
- We recommend to use our AndraBalloon® or Andra2Balloon as Stent-Delivery-Catheter
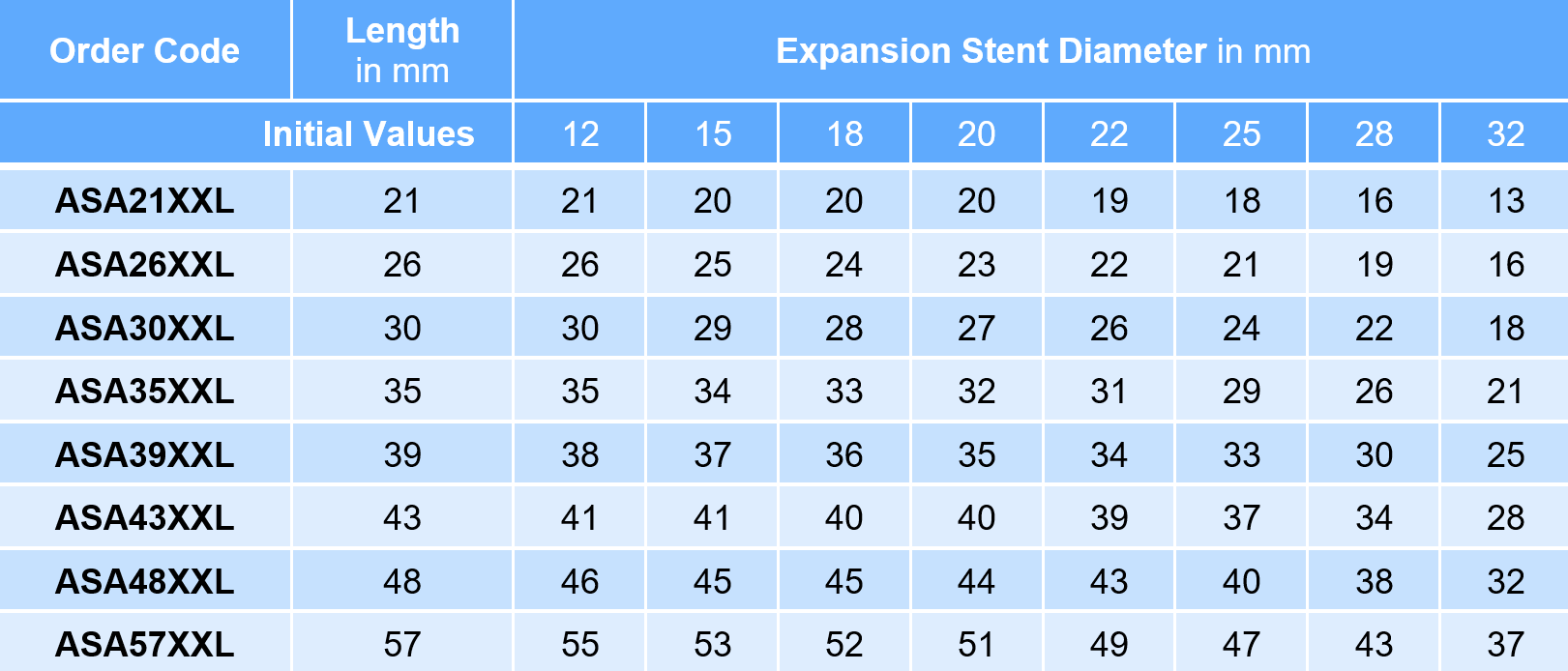
The diameter of the introducer sheath depends on the balloon (+ 1F for the stent).
Packaging unit of the AndraStent® Aortic XXL: 1
 MR Conditional
MR Conditional
Non-clinical testing has demonstrated the AndraStent Aortic and Covered AndraStent Aortic, represented by the Covered AndraStent ASC57XXL are MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 3 Tesla, with
- Maximum spatial field gradient of 7,200 G/cm (72 T/m)
- Maximum force product of 132,000,000 G2/cm (132 T2/m)
- Theoretically estimated maximum whole body averaged (WBA) specific absorption rate (SAR) of 2 W/kg (Normal Operating Mode)
Under the scan conditions defined above, the stent is expected to produce a maximum temperature rise of less than 3.2 °C (2 W/kg, 3 Tesla) RF-related temperature increase with a background temperature increase of around 1.0 °C (2 W/kg, 3 Tesla) after 15 minutes of continuous scanning.
In non-clinical testing, the image artefact caused by the device extends approximately 17.5 mm from the stent when imaged with a gradient echo pulse sequence and a 3 Tesla MR system.
Contact us for more information.
